How iPhone batteries work, and how Apple manages performance over time
Lithium-ion batteries such as those found in nearly every smartphone and tablet are not little, indestructable homogenous power reactors. AppleInsider delves briefly into the construction and chemistry of them, and discusses how an old battery works against not only device manufacturers, but the users themselves.
A battery is a physical and chemical process, and isn't eternal
First, some science 101.
Any battery stores electrical energy in the form of chemical energy, and can convert that energy into electricity. A battery requires an anode and cathode, separated by electrolyte that allows the flow of electrical charge between the cathode and anode.
When the battery is put under load, the anode releases electrons to the negative terminal and ions in the electrolyte through an oxidation reaction. The cathode accepts these electrons, completing the circuit for the flow of electrons.
This is the end of the story for non-rechargeable batteries. However, lithium-ion batteries can be recharged. So, when current is properly applied, the electron flow happens in reverse, recharging the battery.
This is not a linear reaction — also called an "ideal battery." A battery's total capacity measured in milliamp-hours for mobile devices and overall lifetime including charge/discharge cycles are limited by engineering choices made by device manufacturers including charging circuitry and software, plus physical volume of reactants.
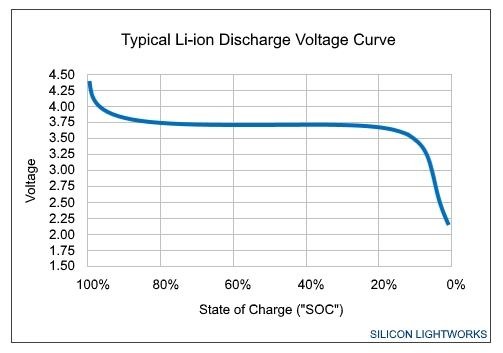
The physically larger the battery, the higher capacity, and the flatter the curve, and the less pronounced the voltage drop is when the battery charge is low. Given the current battery debacle, this is why the Plus-sized iPhones are less affected by slowdowns, and why the iPad doesn't seem to be at all — physically larger batteries.
The better and more regulated the charging and discharging of the cell is, the longer the battery will live. The more temperature excursions the battery endures, like leaving it in a hot car, or outside in freezing temperatures, the shorter the life of the battery will be.
The output voltage of any given battery over a discharge cycle can be plotted over percent charge.
The reactants aren't eternal, though. In the case of a lithium-ion battery, "metal whiskers" can form in the cell, shorting out afflicted portions of the battery cell and cutting down on available power. Ultimately, the whisker formation, coupled with reactant depletion lead to a completely dead battery from under-voltage and non-reversible oxidation.
The slope of the output voltage graph varies
The voltage "curve" and slope varies a great deal based on a number of factors. Battery wear shrinks the horizontal axis. Obviously, device power demand decreases the amount of time it takes to progress along the curve on any given charge.
Damage to the cell from the environment, faulty charging gear providing more than allowable voltage, or other issues permanently increases the steepness of the voltage drop while the battery is being used. Operating temperature has a temporary increase in steepness as well, with low temperature having more of an impact on capacity than high.
A "dead" battery, really isn't
Just because your battery is "dead" doesn't mean that it doesn't have any stored power. This is evidenced by Apple popping up a "plug in" graphic when you try to start a phone with a drained battery.
In the case of a lithium-ion battery, the battery must have more than 2V capacity, or the electrode starts to oxidize. This happens fairly quickly and cannot be reversed by recharging. This is why a lithium-ion battery left idle for a period of time ultimately completely dies, or has next to no capacity.
In all likelihood, the reports of the Samsung Note 8 shutting down and not coming back to life after a drained battery are because the device is below the power that it can kick-start the charging circuitry, and not permanent damage to the battery.
Device manufacturers, and the curve
Without delving too deeply into battery manufacture, device manufacturers have to make choices based on the performance curve. Battery capacity and output voltage are two different things that have to be considered, to say nothing of safety factors.
Every device has a critical voltage, at which point it won't stay operational, may lose data, or crash entirely. The critical voltage is universal, varies from device to device, and is not just applicable to Apple.
Grossly simplified, manufacturers have to look at the battery performance curve, and pick a point that it is making engineering choices for. If they miss their guess, then as a device's battery chemically ages, it will shut down. Yes, this does happen in Android devices — with the Nexus 6P from Sept 2015 now seemingly affected by the problem — and no software fix to prevent the shutdown in sight.
If a lithium-ion battery is compromised by overcharge, overheating, damage, or simply age, the inner cells can "outgas" the contained flammable electrolyte mixture. An undamaged battery membrane will stretch and bulge to contain this material to some extent, but at some point the membrane will rupture explosively — as practically demonstrated by the fires caused by the Galaxy Note 7 in 2016.
How we got here
Mobile processors don't consume a set amount of power. They draw more volts depending on how hard they are being worked.
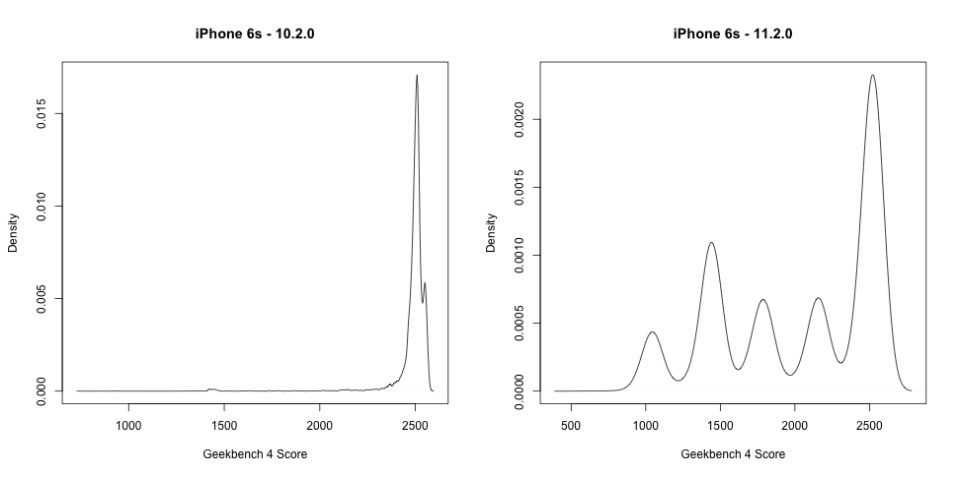
Benchmarking applications are designed to run a processor for as long as it can, as hard as it can to complete a calculation, or do a series of them in an set period of time. This will invariably engage any voltage-related down-clock routine as the processor is slammed by the testing — and it is how Apple's specific method of fixing the random shut-down problem in a voltage-constrained situation was discovered first on Reddit, and then by Geekbench curator John Poole.
It isn't clear how many users actually experienced or noticed the slow-downs, but the random shut-downs before iOS 10.2.1 were certainly noticeable. According to Apple, the slow-downs aren't permanent, and are only invoked with the situation demands it. A battery less chemically depleted will down-clock less often, and a user not pushing the device hard may never see it, even with a near-defunct battery.
There are two different situations surrounding Apple's batteries in the iPhone 6s, and they are unrelated. The first is the battery replacement program that Apple executed for a batch of batteries installed in select iPhone 6s units. In that case, the battery had known manufacturing issues, and Apple replaced them free of charge to owners of the afflicted serial number range.
The second is the shutdown fix it implemented in Feb. 2017 with the release of iOS 10.2.1. What it did was temporarily down-clock the CPU in the phone, to keep the device's voltage demand less than the critical voltage where the phone would shut down seemingly at random. The iOS 10.2.1 is often conflated with the recalled batch of batteries for the iPhone 6s, when in reality, they have nothing in common.
To fix the problem, Apple was less transparent than it should have been, perhaps, in its very brief release notes saying that it had fixed the random shutdown problem. The aforementioned Nexus 6P that looks to be having the same low-voltage problem probably won't get a patch preventing the random crashes.
But, no matter how much money Apple makes, it can't escape the laws of physics, nor the reality of chemistry.
 Mike Wuerthele
Mike Wuerthele










 Malcolm Owen
Malcolm Owen
 Amber Neely
Amber Neely

 William Gallagher
William Gallagher











52 Comments
Very informative. Thanks 😉
This is the best article I've read so far. It's nice to get more information. Keep up the good work.
I'm wondering if the "critical voltage" is higher on the iPhones 6 and later (vs. the 5s and earlier) because that's when the issue started. Maybe newer processors, being more powerful, need a higher critical voltage. Also, iPhone batteries tend to be smaller (iPhones being more power efficient) than Android batteries, and bigger batteries are less affected, so I'm wondering if this is less of a problem in the Android world.
Important info for anyone worried or confused by the battery controversy. The average mobile phone customer (iPhone or Android) probably knows that the battery capacity will decrease over time, but that's about it. Apple wasn't doing anything underhanded with the 10.2.1 update.