Apple's introduction of CoreBluetooth support in watchOS 4 heralded easier glucose control possibilities for diabetics and is a "game changer" according to Dexcom CEO Kevin Sayer, but an Apple partnership with the lead monitor vendor does not appear to be in the cards.
In an interview published by 9to5Mac on Tuesday, the site queried Sayer about the inclusion of CoreBluetooth in watchOS 4, how it pertains to diabetics, and any future possibilities in the field.
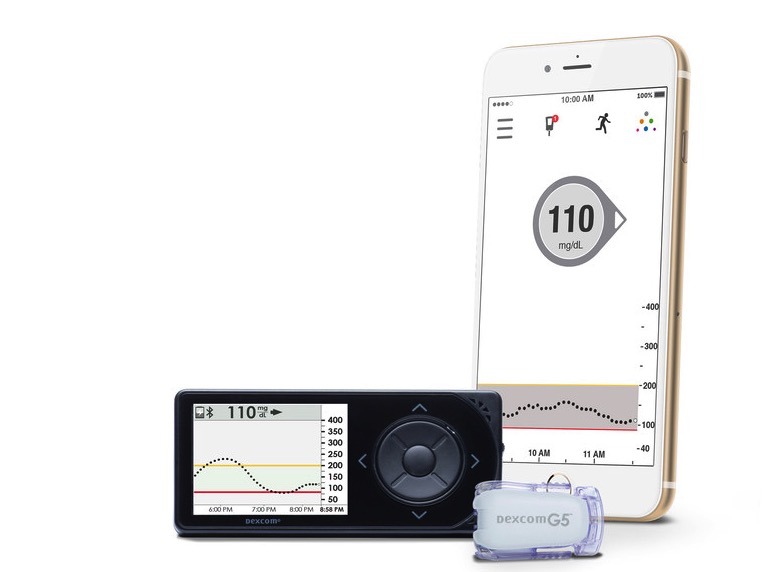
CoreBluetooth is a newer communications protocol than used in previous versions of the Apple Watch. However, it has been a feature of the iPhone for some time. Users needing a continuous glucose monitor, as well as an insulin pump will be able to leave the phone behind after the Apple Watch software is upgraded, needing only the smaller wearable.
"If they have an Apple Watch [with CoreBluetooth support], they can leave that phone home, and track their workout real-time," said Sayer. "There's a number of places where this direct connection will be useful. I think it's part of a much longer term strategy to make that wearable more useful in the lives of people who use it."
Sayer emphasized that with the G5 Continuous Glucose Monitor by Dexcom, that the iPhone would be needed for initial setup at first, and some follow-up data entry.
No magic bullet for monitoring
Sayer discussed the current technology, and reiterated that it was still invasive, if less so than previous generations of the technology. At present, the sensor is a small wire about the same width as a human hair that is inserted just below the skin, and that performs the glucose measurement. He dismissed the possibility of an immediate deployment of external sensors being able to do the measurement, as accurate information is demanded at all times.
"People who intensively manage their diabetes really require extremely accurate information, because insulin is a life saving and a life threatening drug," said Sayer. "If somebody could solve that problem it would be a boon for [diabetics], but we haven't seen anything that's better than what we have and we know how hard it is to do what we do."
The executive did note that technology is constantly advancing, and that the company is experimenting with a number of new form factors. An "ultimate goal" is to make the wearable about the size of a penny, and be able to be worn like a band-aid, or attached to an Apple Watch.
"If it can communicate directly to an Apple Watch that's a wonderful solution for a patient, they'll be thrilled," said Sayer about the future technology. "If Apple asked for a collaboration, we'd certainly talk about it."
What's immediately on the horizon
The Gen 6 Continuous Glucose Monitor is next for Dexcom. It will ship in in 2018, and be a "lower profile" than the G5, with an automated insertion system for the probe.
"The sensor we believe is really the next step in our technology evolution as far as making these things more accurate and more consistent," described Sayer. "Apple users have that to look forward to next year sometime, we hope to file it with the FDA in September."
According to Diabetes Daily, the Gen 6 will be labeled for 10-day continuous use, up from 7. As an improvement to the technology, acetaminophen usage will no longer interfere with the data.
The company is hoping for Medicare coverage of the device at some point in 2018.
The path leading up to this point
In April, a report from CNBC claimed that Apple was working on breakthrough glucose sensors for the Apple watch. The report cited hires of biomedical engineers, with the ultimate goal of producing an integrated Apple Watch monitoring system.
The technology was said to include optical sensors capable of measuring indications of glucose in the blood stream through a user's skin. About 30 people were said to be part of the engineering team a year ago.
In May, just two weeks before WWDC, another report from CNBC circulated that Apple CEO Tim Cook was wearing an "unannounced Apple Watch accessory" to track blood glucose readings. The report was thought to be collaborated by remarks that Cook made in February, with the executive explicitly saying that he had been wearing a monitor for a "a few weeks."
While the CoreBluetooth functionality that will be added to WatchOS 4 for existing devices, if history is any indication, a "Series 3" Apple Watch could arrive this September, alongside an anticipated revamp of the iPhone lineup, headlined by a completely redesigned "iPhone 8" model. In 2016, the Apple Watch Series 1 and Series 2 debuted alongside the iPhone 7 and iPhone 7 Plus.
 Mike Wuerthele
Mike Wuerthele







-m.jpg)






 Andrew Orr
Andrew Orr
 Amber Neely
Amber Neely
 Marko Zivkovic
Marko Zivkovic
 William Gallagher and Mike Wuerthele
William Gallagher and Mike Wuerthele











3 Comments
Consumer glucose monitors using strips and drops of blood have a 20% ± error.
That amount of error is apparently acceptable to the FDA.
If Apple can get within this error rate, then the iPhone glucose monitor is GOLDEN.
I use the current Deacon G4, wear it for 21 days(!), not just 7, and it is a vital part of my disease management, and it 'talks' to my pump. When the battery dies it costs $900 for the sensor replacement, money that is very hard to come by. Anxiously awaiting an Apple solution!
Separating the sensor from the interpretation and display of the data could dramatically lower prices for these devices...
An analogy might be heart rate chest straps like the Polar H7: You can buy one for $75 and all it does is collect the data and send it over to the IPhone for an app to interpret, analyze and display the data. If you had to buy the complete package, that $75 would probably add one or two zeros to the cost.