A report on Friday reveals high-ranking Apple executives met with FDA Commissioner Margaret A. Hamburg and others in December over "mobile medical applications," strongly hinting that a so-called iWatch with health-centric features is well into development.
As noted by The New York Times, senior Apple executives discussed medical electronics with Food and Drug Administration officials on Dec. 13 of last year, though the details and outcome of the meeting are unknown.
According to the FDA's public calendar listing, Apple was represented by SVP of Operations Jeff Williams, VP of Software and Technology Bud Tribble, Michael O'Reilly and government affairs counsel Tim Powderly.
It was reported on Thursday that O'Reilly was hired some time last year in an undisclosed role, though the executive's pedigree is in medical devices. Before moving to Apple, O'Reilly held the position of chief medical officer and executive vice president of medical affairs at pulse oximeter firm Masimo Corporation. He currently currently teaches anesthesiology at the University of Michigan and the University of California, Irvine.
Attending the meeting for the FDA was Jeff Shuren, director of the body's Center for Devices and Radiological Health, and Bakul Patel. The publication described Patel as a "staunch advocate" for patient safety, noting that he drafted the agency's mobile medical app guidelines.
While the reason for the meeting is unknown, Mark A. McAndrew, who spotted the meeting and is a partner at law firm Taft Stettinius & Hollister which handles health and science issues, said the high-level meet-up was anything but ordinary. McAndrew speculates Apple may either be looking for ways to clear regulatory hurdles ahead of a medical device or app launch, or is trying to push something through the FDA after seeing setbacks.
Most interesting is O'Reilly's presence at the meeting. Being a fairly recent hire, his attendance suggests at least one topic of discussion was a device capable of reading a user's pulse rate. Alternatively, Apple may have selected O'Reilly to head up the iWatch team.
Apple is widely rumored to be developing a smartwatch that some expect will have built-in health monitoring capabilities, along with other on-the-go applications. Most wearable devices on the market act as an accessory and connect with a smartphone or other device. Apple's solution is thought to work in much the same way; gathering information for specific iPhone or iPad apps, while serving as a remote screen for viewing of emails, messages and other correspondence.
 AppleInsider Staff
AppleInsider Staff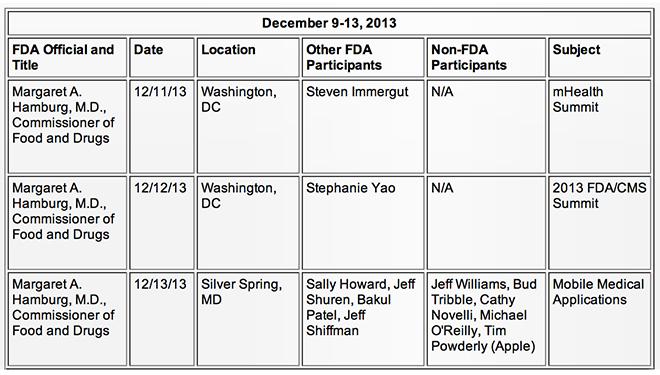







-m.jpg)






 Marko Zivkovic
Marko Zivkovic
 Christine McKee
Christine McKee
 Andrew Orr
Andrew Orr
 Andrew O'Hara
Andrew O'Hara
 William Gallagher
William Gallagher

 Mike Wuerthele
Mike Wuerthele
 Bon Adamson
Bon Adamson




-m.jpg)



23 Comments
The Apple way, not just a watch but something much more. Whatever it is, it has been in development for years, the medical aspect may come from an idea Steve Jobs had while sitting in the hospital when he initally got sick.
That's one disadvantage of developing a medical device: so much regulatory approval is required that Apple won't be able to maintain their usual secrecy.
But yeah the evidence is stacking so high now for *something* medical to come from Apple, I am really curious to see what it is.
I wonder if there's a way, through monitoring of pulse etc, to "optimise" an amount of exercise?
McAndrew speculates Apple may either be looking for ways to clear regulatory hurdles ahead of a medical device or app launch, or is trying to push something through the FDA after seeing setbacks.
I have some experience in preparing documents needed to gain FDA approval regarding medical diagnostic equipment and it has always been pretty smooth. I suppose it gets easier once you have received approvals for similar products in the past. Now we just send them a nice glossy brochure with some forms and filings. The equipment does not have to be evaluated physically or even exist in a production version. FDA is a piece of cake compared to Canada.
That's one disadvantage of developing a medical device: so much regulatory approval is required that Apple won't be able to maintain their usual secrecy.
But yeah the evidence is stacking so high now for *something* medical to come from Apple, I am really curious to see what it is.
I wonder if there's a way, through monitoring of pulse etc, to "optimise" an amount of exercise?
Well the whole objective of CrossFit is to keep your heart rate at a certain level, which is supposed to burn fat and be a better overall workout, so I'm assuming through pulse, you can find the 'right' heart rate to be working out at.
Well the whole objective of CrossFit is to keep your heart rate at a certain level, which is supposed to burn fat and be a better overall workout, so I'm assuming through pulse, you can find the 'right' heart rate to be working out at.
I have never done CrossFit but it sounds like a CrossFit app could make good use of these new sensors.