An app for Abbott's FreeStyle Libre glucose reader is said to be the first third-party iOS title to make use of the NFC chip in Apple's iPhone, previously reserved only for Apple Pay.
To sync glucose readings, users only have to hold their iPhone against the Libre, according to Abbott. The app doesn't yet appear to be in the U.S. App Store, and was spotted by iPhone-ticker.de.
With iOS 11 Apple introduced Core NFC, allowing third-party iPhone apps to scan NDEF (NFC Data Exchange Format) tags. Notably the technology also requires an iPhone 7 or better, despite NFC chips being in iPhones as old as 2014's iPhone 6.
Access to NFC has been a contentious point with developers, some of whom are accustomed to the relatively open support on Android devices. Indeed Australian banks fought a battle to offer their own digital wallets through NFC, but Apple resisted and was ultimately supported by the Australian Competition and Consumer Commission.
 Roger Fingas
Roger Fingas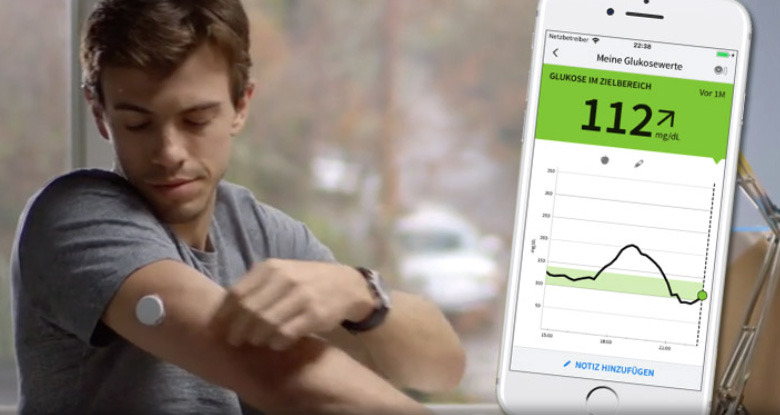








 Christine McKee
Christine McKee
 Malcolm Owen
Malcolm Owen
 Marko Zivkovic
Marko Zivkovic

 Andrew Orr
Andrew Orr
 Andrew O'Hara
Andrew O'Hara
 William Gallagher
William Gallagher



-m.jpg)



11 Comments
As we all know, being “open” carries a lot of responsibility when dealing with security components, something many developers could care less about, especially android developers not used to worrying about any restrictions or security.
This warning from the manufacturer’s site makes me wonder if this isn’t just a novelty. After all, the entire point of checking finger-stick blood glucose levels is to get real-time results so that insulin can be dosed, or for a glucose log so that your doctor can adjust your oral medications.
https://www.myfreestyle.com/provider/?source=www.freestylelibre.us
I suppose that if the glucose values recorded are not real-time, but are accurate, they may be sufficient for someone that is not taking insulin, but even so, the manufacturer says to continue the finger-sticks while using this system.
And...
Note the “aiding in the detection of ... hypoglycemia”. Now another quote.
Inaccurate 40% of the time! That’s almost as bad as flipping a coin (50%), so it cant be trusted for hypoglycemia monitoring. Hopefully the hyperglycemia monitoring is more accurate.
So in summary, you can use this device, but you still have to do the finger-sticks, and the device may be inaccurate. Sounds like they have a long ways to go.
The first challenge its FDA approval. If they have that then it can be assumed that the device will be somewhat reliable. The second challenge is insurance (including Medicare and Medicaid) accepting and paying for the device and supplies.
The competition is going to push for improvements in performance/comfort of the entire market.
Overall I happy and will wait to see how things shake out (including linking with the Apple Watch) before investing.
It's been approved and used in Europe for a a couple of years now. I'm pretty sure FDA approval is around the corner. Medicare/Medicaid coverage is going to be a different story, as it's a significant cost increase over the lancet/test strip combo, so I assume in the beginning it will only be covered for special cases (children, people on blood thinners, etc...) and eventually be extended to all as production ramps up and costs go down. An amazing product and it's fantastic that they were able to work with Apple to access NFC. I know they've been supporting Android for a long time but required an external reader for iPhone, that was very disappointing.