The FDA has approved the Apple Watch's atrial fibrillation history feature under its stringent Medical Device Development Tools program that specifies what devices health professionals can rely on.
Apple Watch has had an atrial fibrillation feature since 2022, and the FDA approved its use just hours before Apple announced it. Since then, it has been credited with saving lives, including those of people who previously had no reason to suspect they had severe heart problems.
Now on top of its allowing the sale of the feature, the Food and Drug Administration has also passed the Apple Watch's AFib history feature for its Medical Device Development Tools (MDDT) program. As first spotted by MyHealthyApple, this makes the Apple Watch the first digital health technology to qualify under MDDT for a non-invasive way of estimating atrial fibrillation.
According to the FDA, the MDDT program is for it to "qualify tools that medical device sponsors can choose to use in the development and evaluation of medical devices."
Apple submitted the Apple Watch atrial fibrillation history feature to the FDA on December 21, 2023, according to an MDDT filing. As part of the application, Apple supplied data from a clinical study of 280 people where the Apple Watch's results were compared to a previously-qualified reference device.
"The average difference between the AFib History Feature and the reference device's weekly burden estimate was 0.67% with a 95% confidence interval of (-0.05%, 1.38%)," says the FDA filing. "92.9% (260/280) of subjects had paired weekly AFib burden differences within 5%; 95.7% (268/280) of subjects' weekly AFib burden estimates were within +10% of the reference."
Noting that medical device developers "have already incorporated [the Apple Watch]... within clinical studies," the FDA concludes that Apple's device qualifies for the MDDT program. However, because the Apple Watch does not identify atrial flutter or atrial tachycardia, the FDA says it is qualified "only as a secondary endpoint to compare estimates of AFib burden."
As well as specifically alerting many users to atrial fibrillation, the feature has led patients to be diagnosed with diabetes.
 William Gallagher
William Gallagher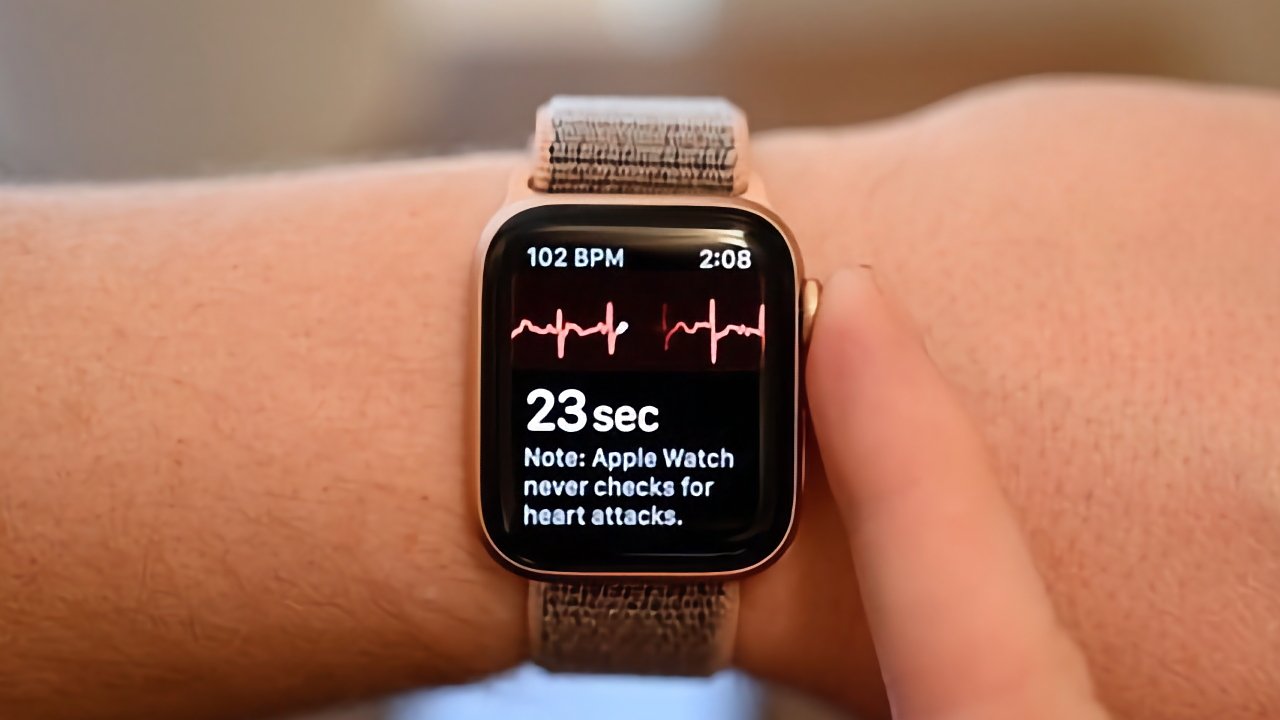













 Wesley Hilliard
Wesley Hilliard
 AppleInsider Staff
AppleInsider Staff
 Andrew Orr
Andrew Orr
 Amber Neely
Amber Neely











4 Comments
Amazing Apple. Thank you for saving lives.
I don't know, something must be wrong with me, as I don't EVER have the Apple Watch flag me as in AFIB, but if I run the heart monitor THEN it will tell me I am in AFIB, assuming it does detect it actually. MOST of the time when I feel my heart going wrong I get the "Inconclusive" reading message instead:

*** of course, none of this matters with newly-sold devices from Apple right now -- two paragraphs about Masimo's ban ongoing ***
Is this true? From my understanding, the Masimo ban has only resulted in the disabling of the pulse oximetry feature--i.e., blood oxygen level--on the Apple Watch. It does not, afaik, affect the ECG and heart-rate tracking functions that are key in detecting AFib.
Also: I'm surprised that Apple Insider has not kept up with the latest Masimo news. First, their consumer products divisions--in healthcare and, ridiculously, in higher-end audio brands--are up for sale. They are looking to focus exclusively on hospital-grade medical equipment which has always been their profit center. So much for Masimo's efforts in the consumer watch business beyond the W1. And second, activist investors in Masimo are looking to oust CEO Joe Kiani, who spent a fortune suing Apple with nothing to show for it beyond the current, U.S.-only disabling of pulse oximetry on Watch 9 models sold in 2024... then spent another fortune acquiring Sound United, the home of eight higher end audio brands like Bowers & Wilkerson and Marantz... and who has now taken out a giant loan against the shares he owns in Masimo, so he can maintain his pecentage of ownership in the company while having money to live on during this proxy battle. The vast majority of companies don't permit that kind of borrowing against shares because of the potential for serious negative consequences, but Masimo isn't one of those companies.