Apple has been picked as one of nine companies for a pilot program by the U.S. Food and Drug Administration, which which will let the firms avoid regulations that can slow the development of health-related products.
Under the scheme, companies will be able to get pre-clearance so long as they subject their software and facilities to scrutiny, Bloomberg reported. With this in hand, the formal approval process may become shortened or non-existent.
Over a hundred companies expressed interest in the pilot, the FDA said. Other successful applicants included Fitbit, Samsung, Roche, Johnson & Johnson, and Alphabet's Verily Life Sciences.
In the case of Apple, the FDA program could be applied to things like the Apple Heart Study, but also future versions of the Apple Watch. Apple has allegedly been experimenting with non-invasive glucose detection, something that would require FDA vetting for a shipping product.
Going through the FDA has previously posed risks for the company, not just because of its time-consuming nature but the likelhood of exposing products to competitors long before they launch. While secrets are still likely to slip out, rapid approval could make it possible for Apple to ship medical-related advances before competitors can catch up.
The Apple Watch Series 3 shipped on Sept. 22 with upgrades like LTE, but no radically different health technology.
 Roger Fingas
Roger Fingas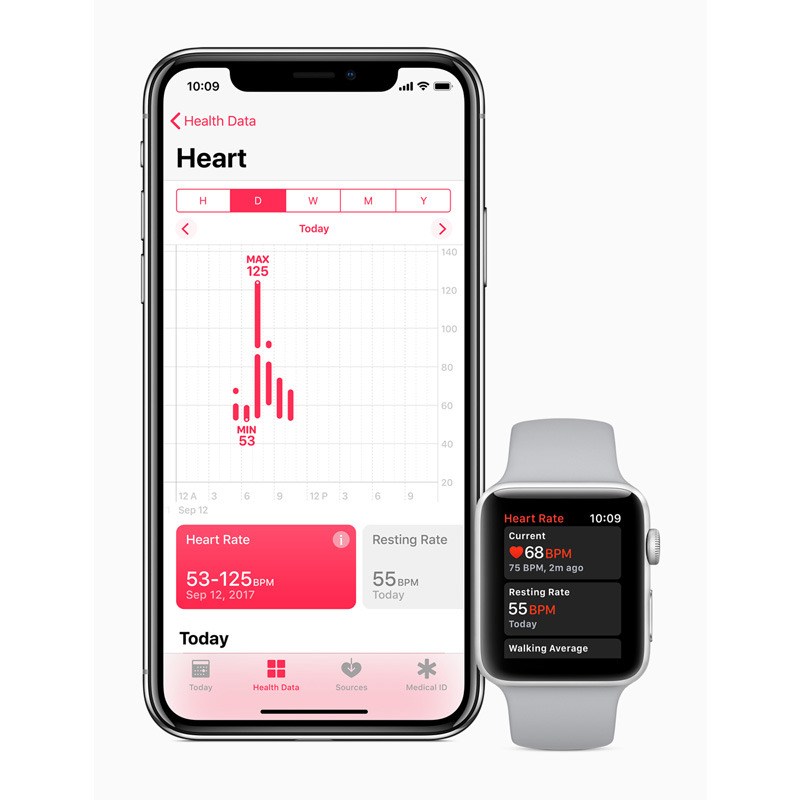








 Brian Patterson
Brian Patterson
 Charles Martin
Charles Martin


 Malcolm Owen
Malcolm Owen
 William Gallagher
William Gallagher
 Christine McKee
Christine McKee
 Marko Zivkovic
Marko Zivkovic








14 Comments
Wait. What? Why? Regulation exists for very real and important reasons. Why would they just let certain companies slip by with less or zero regulatory process??
I predict that, unfortunately, the FDA is going the way of the EPA and gradually losing its regularly enforcement power.
I think this is related to the administration in power. Trump is very much pro corporation. .