The Apple Heart Study, conducted in partnership with Stanford Medicine, collected heart rate data from more than 400,000 Apple Watch users in its attempt to determine whether wearable devices can effectively detect irregular heart rhythms. Contrary to previous reports, however, the results were not used to gain clearance from the U.S. Food and Drug Administration for Apple Watch Series 4's ECG feature.
Stanford revealed the scope of the Apple Heart Study in an announcement Thursday, saying the clinical trial was the largest screening study on atrial fibrillation ever conducted. A paper detailing the study and its design was published in the American Heart Journal today.
Apple closed enrollment in August, some eight months after it launched the program in 2017. Participants began receiving word that the study was complete in September, though Stanford says data collection will be completed early next year, in line with previous statements from Apple.
Following the closure to enrollment, Apple quietly submitted a de novo request for FDA clearance to two Apple Watch apps that would feature prominently in Apple Watch Series 4. The first app handles interpretation of and display of electrocardiogram readings from the wearable's new ECG system, while a second uses optical sensors to identify irregular heart rhythms.
The FDA issued regulatory Class II clearances — over-the-counter access — for both.
Previous reports claimed Apple leveraged the Heart Study in both de novo requests, but Stanford says the trial was used only in respect to atrial fibrillation notifications.
The clarification makes sense, as the Heart Study related to atrial fibrillation, not ECG systems or data collection. During the months-long trial, a specialized app collected pulse rate data from Apple Watch Series 1, 2 and 3 hardware. In some cases, the app was able to detect and notify users of irregular pulse rate episodes.
The study, according to Stanford, sought to determine how many patients who received irregular pulse notifications were found to have atrial fibrillation, and how many went on to get medical attention. Calculating the accuracy of the system against simultaneous ECG recordings was a tertiary goal of the trial.
Though it did not factor into regulatory approval of Apple's ECG solution as previously thought, the Heart Study can be considered an important first step toward providing consumers with easily accessible medical hardware.
"We now have access to high-quality sensors that can measure and detect changes in our bodies in entirely new and insightful ways without even needing to go to the doctor, but we need to rigorously evaluate them," said Mintu Turakhia, MD, associate professor of cardiovascular medicine at Stanford. "There's never really been a study like this done before."
Apple is expected to activate the ECG feature in Apple Watch Series 4 later this year.
 Mikey Campbell
Mikey Campbell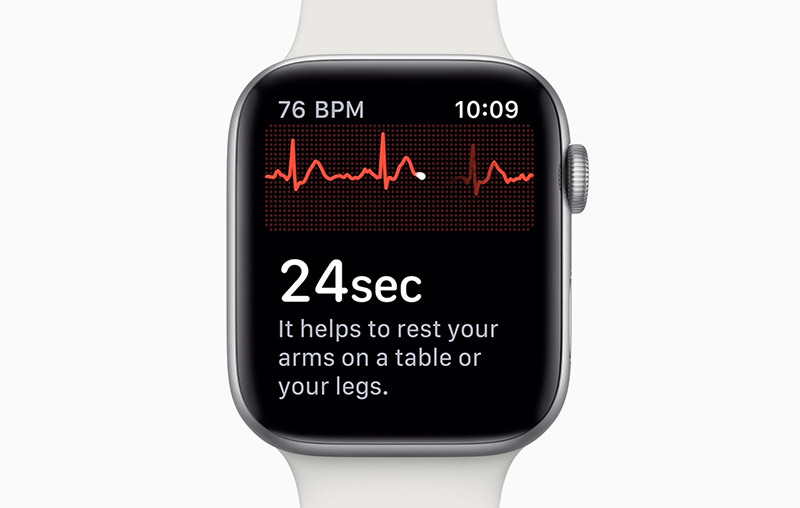

-m.jpg)






 Thomas Sibilly
Thomas Sibilly
 Andrew O'Hara
Andrew O'Hara
 Amber Neely
Amber Neely
 Marko Zivkovic
Marko Zivkovic
 Malcolm Owen
Malcolm Owen
 William Gallagher and Mike Wuerthele
William Gallagher and Mike Wuerthele










11 Comments
Good to hear, I'm glad it had purely for scientific motivations.
....and when can we expect the release, not just in the US but other countries?
I was wondering how that study would have had bearing on the ECG function. Sure the AW's ECG feature can help sniff out AF, but the heart study was testing with a different set of sensors, it wouldn't have been relevant for getting the OTC approval for the ECG function.
Also about those OTC FDA approvals. While receiving FDA approval is a sign of the ECG feature having a high degree of accuracy, certain consumer medical devices can be approved even if there is a chance they could provide imperfect information. The FDA already approves a range of devices which have varying degrees of accuracy, as they suit different use cases. A single-lead ECG Apple Watch has significant benefits over the risks.
A good parallel to think about is the home pregnancy test kit. Yes it can provide a false positive, but it's allowable because the risks associated with false positives are low and it keeps medical costs down while unclogging the system for more urgent care. Those with a positive result can seek a medical professional for higher certainty in a single appointment, rather than an appointment every time there are symptoms.
The same applies with the ECG - it's going to help reduce the workload on medical professionals, especially as irregular heart behaviours can't be reproduced on command in the window of a doctor's appointment and that other symptoms(such as muscular spasms or digestion) can feel like issues with the heart.