The announcement, made earlier today, gives a green light for Mobile MIM, an iOS app component of secure medical imaging product sold by the Cleveland-based MIM Software.
The FDA said the app "is not intended to replace full workstations and is indicated for use only when there is no access to a workstation," but William Maisel, MD, MPH, the chief scientist and deputy director for science in the FDA’s Center for Devices and Radiological Health, noted that "this important mobile technology provides physicians with the ability to immediately view images and make diagnoses without having to be back at the workstation or wait for film."
The Mobile MIM app allows radiological images to be securely delivered to mobile doctors using an iPad or iPhone, enabling them to view images and make medical diagnoses "based on computed tomography (CT), magnetic resonance imaging (MRI), and nuclear medicine technology, such as positron emission tomography (PET)."
The app "allows the physician to measure distance on the image and image intensity values and display measurement lines, annotations and regions of interest," the report stated.
A lengthy evaluation
"In its evaluation, the FDA reviewed performance test results on various portable devices," the agency said. "These tests measured luminance, image quality (resolution), and noise in accordance with international standards and guidelines. The FDA also reviewed results from demonstration studies with qualified radiologists under different lighting conditions. All participants agreed that the device was sufficient for diagnostic image interpretation under the recommended lighting conditions."
MIM Software's chief technology officer Mark Cain stats on the company's website that "establishing a diagnostic protocol for medical imaging is no simple matter for a device like the iPhone or iPad. It is critical to understand the characteristics of the device and to establish methods and tools that are safe and effective, while working within those constraints. There has been a gap in the market for a remote imaging device like this, and now it can be filled."
The app is available in 14 languages and in 34 countries in addition to the US. It is expected to become available in the App Store next week, according to the company's site.
 Daniel Eran Dilger
Daniel Eran Dilger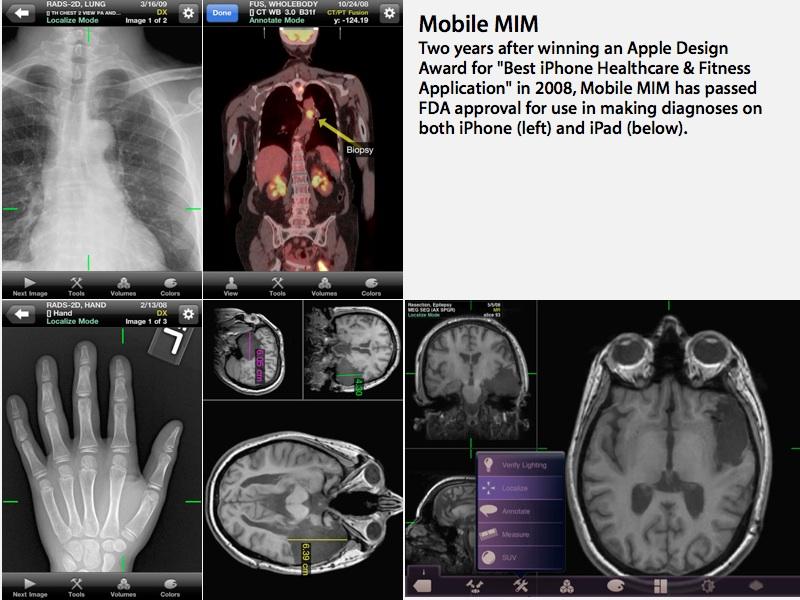






-m.jpg)





 Christine McKee
Christine McKee
 Malcolm Owen
Malcolm Owen
 Amber Neely
Amber Neely



 Chip Loder
Chip Loder








52 Comments
Helps make the iPhone and iPad more useful in the healthcare industry..
iPad is...radiological.
Amazing that it took almost three years to get the product approved. The FDA doesn't mess around (usually) in the healthcare industry. Since it's mainly more a hardware reason than software reason, I wonder if there are Android apps in the works as well. The build quality of those units are much lower.
Absolutely huge. Takes the possibilities associated with this medium to the next level.
The technological constraints, combined with medical complexities involved, are non-trivial. The CTO's statement that it was ".....no simple matter" is spot on.
Kudos to the folks involved, the FDA, and not the least, iPad and iPhone.
Cool!