A pair of new patent applications published this week by the U.S. Patent and Trademark Office and discovered by AppleInsider demonstrate the work Apple has done internally on fuel cells. The filings describe how Apple could build the power sources smaller and lighter for portable devices.
The first application, entitled "Parallel Fuel Stack Architecture," describes how Apple could arrange a set of fuel cells into a fuel stack. In the second filing, named "Reduced-Weight Fuel Cell Plate," Apple describes how it could use lightweight electrically conductive and corrosion-resistant material to build a fuel cell.
The applications explain that fuel cells provide electrical power by converting a fuel, such as hydrogen or a hydrogen-containing compound, into an electric current. Fuel cells contain an anode, a cathode, and an electrolyte between them.
In a fuel cell, a catalyst at the anode oxidizes the fuel and produces positively charged ions and electronics. Ions from the oxidization process then pass through to the cathode while blocking the passage of electrons, and the electrons then drive a load connected to the fuel cell.
For a waste product, the ions recombine with a negatively charged atom, such as oxygen, at the cathode. Any waste from a fuel cell escapes as carbon dioxide and/or water.
A fuel cell typically produces low voltages between 0.5 and 0.7 volts, requiring multiple fuel cells to be combined to create a fuel cell stack. But these stacks come with a number of inherent issues.
For starters, fuel cell stack architectures can have a single point of failure in a connected series. Fuel cells may also fail for a number of reasons, including accumulation of nitrogen in the anode, degradation of the electrolyte, or water flooding in the anode or cathode. Because of this, the reliability of a fuel cell stack can decrease as the number of cells in the stack grows.
Apple's solution for this issue is to build multiple fuel cells connected in a parallel configuration by a power bus, along with a voltage-multiplying circuit to increase the voltage of the stack. In this way, the reliability of the stack would be increased while the fuel cells could also potentially power devices with higher operating voltages.
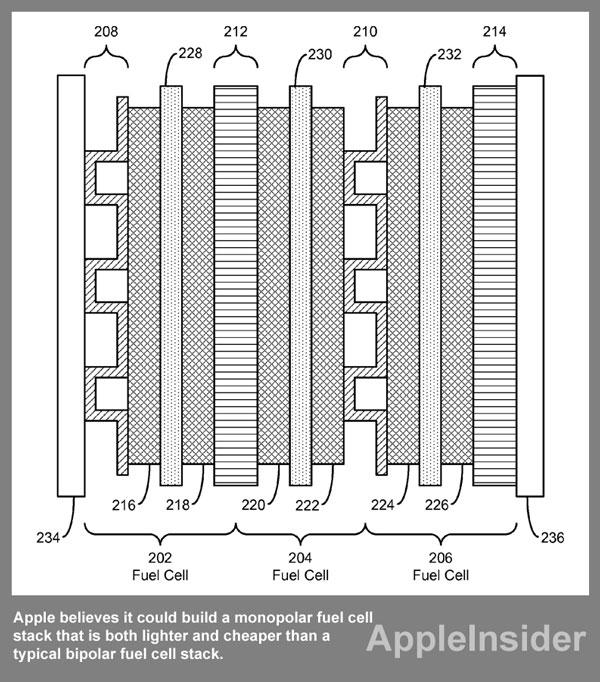
Another problem with fuel cells detailed by Apple is their bipolar plates are typically built with conductive and corrosion-resistant materials, such as stainless steel, that are high in density and add weight to the fuel cells. A stack of cells, all made of stainless steel, can create a power source and portable device that are too heavy to be used practically.
To address this problem, Apple proposes arranging the fuel cells in a monopolar configuration to enable sharing of electrodes between adjacent fuel cells in the fuel stack. This sharing of electrodes could significantly reduce the number of electrodes in the fuel stack, and also enable the use of monopolar plates that are lighter and thinner.
In this method, Apple believes it could build a monopolar fuel cell stack that is both lighter and cheaper than a typical bipolar fuel cell stack. Even with the reduction in weight and cost, the filing says the stack could contain the same number of fuel cells, or even be more powerful than a traditional bipolar fuel cell stack of the same size.
Both patent applications, made public this week, were first filed with the USPTO in April of 2010. The parallel architecture filing is credited to Steven. C. Michalske and Bradley L. Spare, while the reduced weight application is credited to Vijay M. Iyer, Jean L. Lee and Gregory L. Tice.
Apple has frequently explored the possible use of alternative energy sources in its devices to make them more efficient and environmentally friendly. While the mention of fuel cells in an application from Apple is unique, the company has repeatedly (1, 2, 3) explored the option of solar power in its portable electronics.
 Neil Hughes
Neil Hughes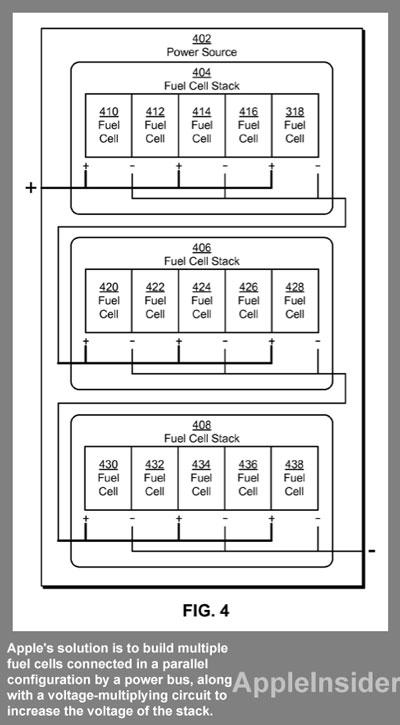






-m.jpg)




 Charles Martin
Charles Martin
 Christine McKee
Christine McKee
 Wesley Hilliard
Wesley Hilliard
 Malcolm Owen
Malcolm Owen
 Andrew Orr
Andrew Orr
 William Gallagher
William Gallagher
 Sponsored Content
Sponsored Content








39 Comments
Remember the liquid metal patent Apple was granted not so long ago? Well, it was about fuel cells, and I can only assume that these patents have something to do with each other.
It might be an exciting time for LQMT-stock owners
Hydrogen isn't a green technology whatsoever. It is inefficient, because energy is lost as it is transferred from other sources of energy.
Hydrogen isn't a green technology whatsoever. It is inefficient, because energy is lost as it is transferred from other sources of energy.
energy is always lost, the question is how much energy.
Hydrogen has been a pipe dream and means by which to keep an oil stranglehold on energy since they used it to explain away the discontinuation of GM's EV-1? IN THE EIGHTIES.
I don't believe that any hydrogen fuel cell will ever be mass produced nor hydrogen used for any purpose whatsoever beyond getting stuff into space.
And that's good. Because we don't need worthless tech that takes thirty years to show zero progress. Better electric batteries and more efficient electric motors is what we need.
Apple should design car batteries. Heck, with the advancements in battery tech they've done, we could see driving ranges jump from 200 miles to 700, effectively removing any need for a gas-powered vehicle in 97% of all use cases.
energy is always lost, the question is how much energy.
Yes, but the production of hydrogen adds another often unnecessary step in the chain.
Fossil fuel->electricity->hydrogen->electricity more than often the case.
Hydrogen just irritates me because the green brigade often forget to mention the original source of energy that produces the hydrogen.