Apple Watch Series 4 EKG tech got FDA clearance less than 24 hours before reveal
The electrocardiogram feature in the Apple Watch Series 4 got clearance from the U.S. Food and Drug Administration just a day prior to Apple's Sept. 12 press event, reportedly causing some stress at the company.
The FDA's classification letters to Apple were dated Sept. 11, Fast Company noted on Tuesday. The site added that just after the Sept. 12 press event, it overheard an Apple worker talking about the close timing "in exasperated tones" outside the Steve Jobs Theater.
Had the clearance not been granted, Apple might have had to radically revise COO Jeff Williams' presentation on Sept. 12, in which the EKG technology and its FDA clearance were front and center. Williams claimed that the Series 4 is the "the first EKG product offered over the counter directly to consumers."
Similar technology is already in use by AliveCor, which markets the KardiaBand accessory for Apple Watch. The company reached out to AppleInsider to note that KardiaBand was first to receive over-the-counter FDA clearance in 2014, three years prior to wide general release in November 2017. As CNBC reporter Christina Farr explained in a tweet, however, users must have their first ECG reviewed by a doctor in order to "unlock," or view, the reading.
AliveCor details the process on its website, saying that "due to regulatory necessity, new U.S. accounts are required to have their first EKG reviewed by a U.S. board-certified cardiologist free of charge (a $19 value within the app)."
The Apple Watch, on the other hand, has FDA clearance to show its first ECG to users without a doctor review.
The FDA has technically only cleared an "Irregular Rhythm Notifcation Feature" and EKG software that can detect signs of arrythmias. The FDA wrote that the Watch's EKG feature "is not intended to provide a diagnosis," and "not intended for use by people under 22 years old."
Indeed the Series 4 won't even ship with the EKG app installed when it launches on Sept 21. That's coming in an over-the-air update before the end of 2018.
Apple was likely eager to tout the feature in advance however as a way of differentiating its product not just from rivals like Garmin and Fitbit, but earlier Watch models, since the biggest upgrade is otherwise a slightly larger screen.
 Roger Fingas
Roger Fingas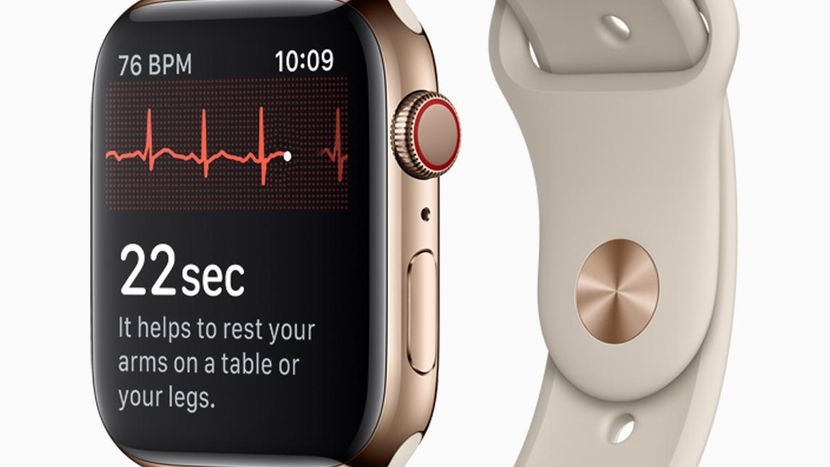











 Amber Neely
Amber Neely
 Thomas Sibilly
Thomas Sibilly
 AppleInsider Staff
AppleInsider Staff
 William Gallagher
William Gallagher
 Malcolm Owen
Malcolm Owen
 Christine McKee
Christine McKee










19 Comments
As coincidence would have it I had an appointment with my cardiologist yesterday. I breached the subject of the Apple Watch and he was well aware of it. He told me the medical community is very interested in this development but that they want much more information on how it works, how accurate it is, etc. He also explained his take on the difference between FDA “clearance” and FDA “approval.” The operative word is treatment. If a device is going to be used to actually perform medical treatment, like say the insulin pumps some diabetics wear, that requires FDA approval. The portable EKG machine in his office does not perform medical treatment but is a diagnostic tool he uses and would only require FDA clearance that it works as designed.
Meanwhile I have read a couple of articles from critics who worry that this technology will cause too many false positives, cause more people to go to the emergency room which could affect the availability of prompt medical care for those who really do have a problem. That kinda makes sense I guess.
Then he proceeded to lecture me once more on losing weight and exercising more often. I’m trying, Doc, I’m trying, but that cheesecake is beckoning me.
I'm pretty sure Apple prepared some Plan B slides that said "FDA clearance expected soon" or the like.