The U.S. Food and Drug Administration has issued preliminary guidance that defines the agency's criteria for determining when wearables which provide health tracking or advice, such as the forthcoming Apple Watch, could be classed as regulated medical devices.
According to the draft — Â which is currently open for a 90-day public comment period — Â wearables will not be considered medical devices unless they make claims about fitness to treat specific diseases or conditions, or present inherent risks to consumers' safety. Among the claims that the FDA will allow under the umbrella of "general wellness" are those related to weight management, physical fitness, relaxation or stress management, mental acuity, self-esteem, sleep management, or sexual function.
Devices which make claims regarding the treatment or diagnosis of diseases or conditions such as obesity, eating disorders, anxiety, autism, muscle atrophy, or erectile dysfunction will not be considered in this category and will face FDA scrutiny.
Additionally, those devices which track activity or biological information, such as the wearer's pulse, may make certain disease-related claims when it generally understood that living a healthy lifestyle will reduce the risk of contracting that disease or aid in its management. For instance, promoting "physical activity, which, as part of a healthy lifestyle, may help reduce the risk of high blood pressure" would be allowed and would not subject that device to regulation.
The FDA also notes that devices which are invasive, pose a risk to the user without sufficient controls (such as lasers), or raise "novel questions of usability" or biocompatibility will not fall under these guidelines.
Few, if any, of the current crop of wearable devices appear to run afoul of these rules. For its part, Apple is known to have met repeatedly with FDA officials in the run-up to the Apple Watch's unveiling, likely to ensure that the device would meet any forthcoming regulatory requirements due to its strong focus on health tracking.
Following the release of iOS 8 and its new Health app, the agency said when asked about the meetings that Apple "wants to make sure they are on the side of the FDA" and that "the earlier FDA is involved and advising, the less likely that Apple would be caught by surprise later when they wish to release a new product, if that product must be regulated."
 Sam Oliver
Sam Oliver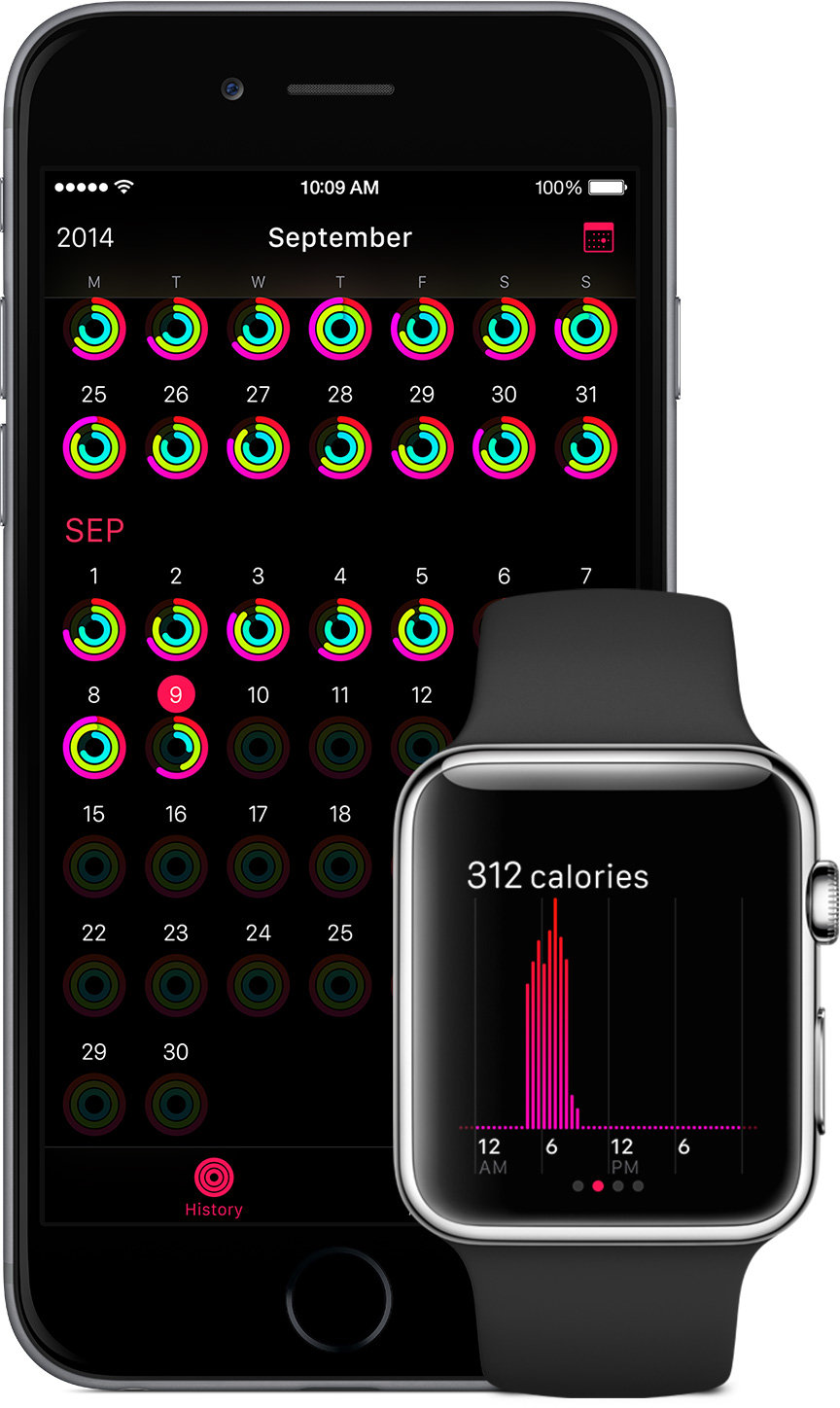











 Amber Neely
Amber Neely
 Andrew O'Hara
Andrew O'Hara
 William Gallagher
William Gallagher

 Christine McKee
Christine McKee
 Andrew Orr
Andrew Orr
 Charles Martin
Charles Martin
 Marko Zivkovic
Marko Zivkovic





34 Comments
I'd rather take medical advice from an AppleWatch than the FDA.
I'd rather take medical advice from an AppleWatch than the FDA.
There is no law against stupidity.
Knock yourself out.
There is no law against stupidity.
Knock yourself out.
Indeed.
If any device is going to be monitoring something important, like insulin levels for example, I want the FDA all over it.
[quote name="anantksundaram" url="/t/184390/with-apple-watch-near-fda-clarifies-regulatory-stance-on-health-tracking-wearables#post_2663642"]There is no law against stupidity. [/quote] Yep. Especially in Internet comments.
[quote name="AppleZilla" url="/t/184390/with-apple-watch-near-fda-clarifies-regulatory-stance-on-health-tracking-wearables#post_2663644"][quote name="anantksundaram" url="/t/184390/with-apple-watch-near-fda-clarifies-regulatory-stance-on-health-tracking-wearables#post_2663642"]There is no law against stupidity. [/quote] Yep. Especially in Internet comments.[/quote] Spot on.