Medtronic gets FDA approval for new iPhone & Apple Watch connected diabetic therapy system
Medtronic has announced that its long-awaited 780G diabetic therapy system has gained FDA approval which includes support for iPhone and Apple Watch monitoring.
The Medtronic 780G system encompasses the insulin therapy pump and the Guardian 4 constant glucose monitor — or CGM. Combined, Medtronic is able to create a hybrid closed-loop delivery system that can issue automatic corrections based on blood sugar.
It also works to make corrections with automatic meal corrections. Users can input their estimated carbs consumed and if the pump detects your blood sugar spiking rapidly, it will issue a stronger correction dosage to bring you within range.
If you forgot to bolus for a meal, it too will deliver a correction bolus to counteract the carbs. Should you drift low, insulin delivery will be suspended.
This is similar to the Tandem T:Slim X2 that works with the Dexcom G6 sensors for its hybrid closed loop system. Soon it will add support for the Dexcom G7.
Like with Dexcom, high and low blood sugar will show on your Apple Watch and iPhone with new readings appearing every five minutes. Unlike Tandem's system, you are unable to control the insulin pump from your phone at this time.
The Medtronic 780G requires a prescription for use. Type I diabetics interested in it can sign up to be notified of its availability on Medtronic's website.
 Andrew O'Hara
Andrew O'Hara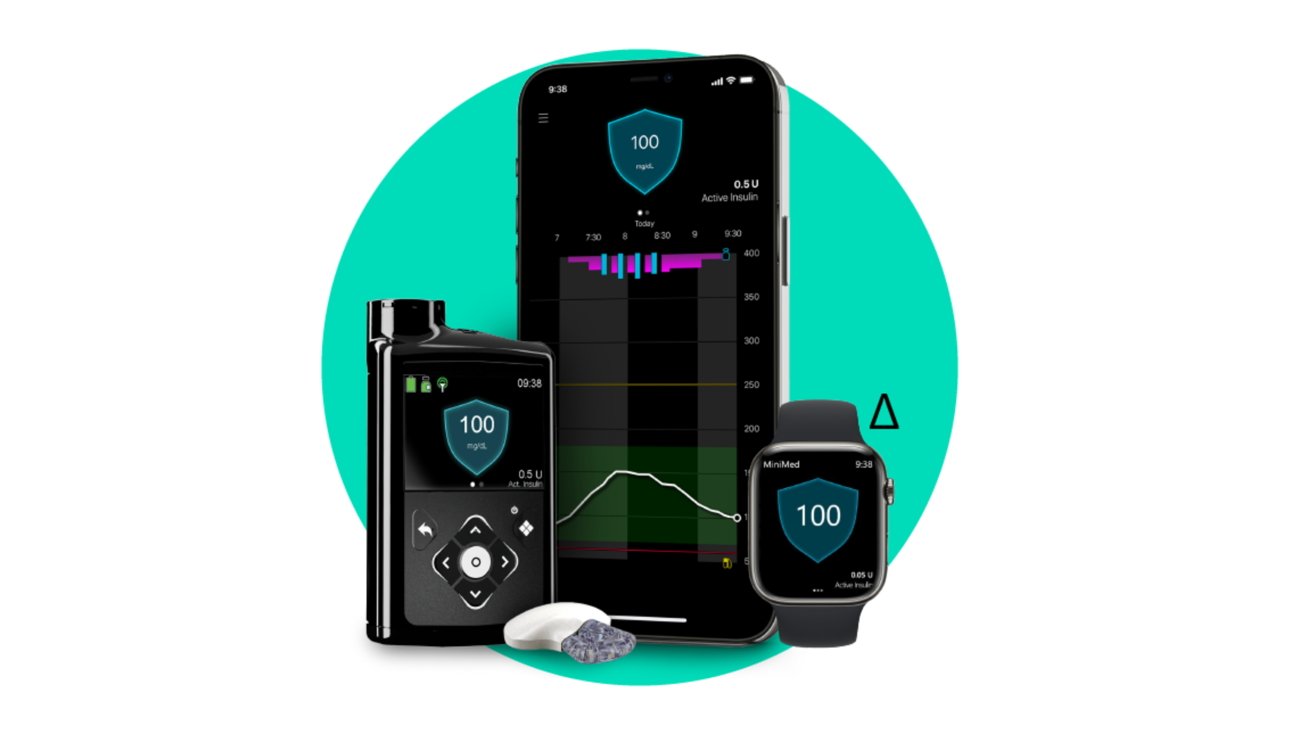






 Amber Neely
Amber Neely
 Andrew Orr
Andrew Orr
 William Gallagher
William Gallagher
 Christine McKee
Christine McKee
 Malcolm Owen
Malcolm Owen











3 Comments
This is potentially life saving. Big news.
Yes fair enough. The more widespread this gets the better. Sadly, this comes too late for my friend…